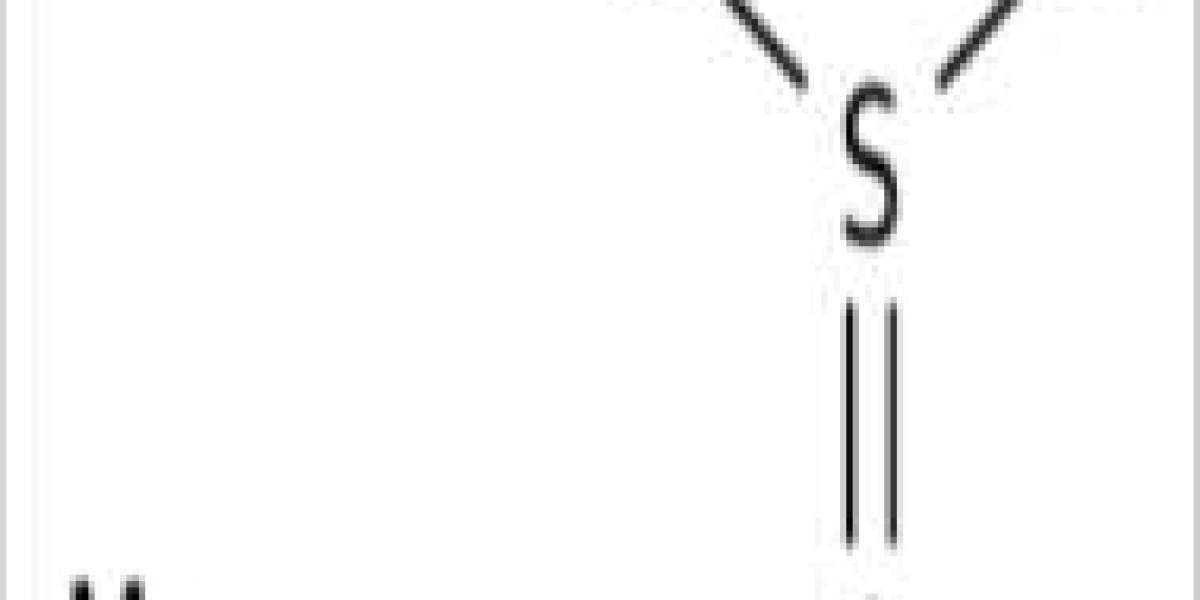
Magnesium sulfite is the magnesium salt of sulfurous acid with the formula MgSO3. Its most common hydrated form has 6 water molecules making it a hexahydrate, MgSO3·6H2O. When heated above 40 °C (104 °F), it is dehydrated to magnesium sulfite trihydrate, or MgSO3·3H2O.[1] The anhydrous form is hygroscopic, meaning that it readily absorbs water from the air.
What are the best productivity hacks, tips, tools or resources for a project manager?
Profile photo for Joshua Zerkel
Literature data of solubility of MgSO3 in water and in aqueous solutions of MgSO4 have been correlated. Magnesium sulfite forms hexahydrate (stable below 40°C) and trihydrate (above 40°C), nevertheless, metastable hexahydrate can precipitate at temperatures significantly higher than this transition temperature. Magnesium sulfate increases the solubility of the sulfite.
Magnesium Sulfite Hexahydrate is hydrated magnesium salt of sulfurous acid, that produced by the reaction of magnesia (magnesium oxide) with sulphur dioxide. The anhydrous form is hygroscopic and it readily absorbs water from the air. Magnesium sulfite forms hexahydrate (stable below 40°C) and trihydrate (above 40°C), nevertheless, metastable hexahydrate can precipitate at temperatures significantly higher than this transition temperature. Magnesium Sulfite Hexahydrate used in the sulphite wood pulping process used in the paper industry, and in magnesia scrubbing of SO2 from flue gases. In both instances magnesium sulphite is used in preference to other similar substances because it is able to regenerate and recycle sulphite in these processes and is therefore a "greener" alternative.
Oxidation of magnesium sulfite is important for recycle of byproduct in the magnesium desulfurization. The oxidation rate of magnesium sulfite, prepared by vacuum evaporation method, was investigated in a bubbling tank in presence of transition metal catalysts, which shows cobalt is the most effective. The general reaction orders with respect to cobalt, magnesium sulfite, and oxygen are 0.44, 0, and 0.46, respectively, and the apparent activity energy is 17.43 KJ·mol. The catalytic performance of cobalt compared with other metals was also analyzed employing the ion potential theory. Integrated with the three-phase reaction model, we inferred that the general oxidation rate of magnesium sulfite is controlled by mass transfer of oxygen. Further, the intrinsic kinetics was predicted, indicating that the reaction orders with respect to cobalt and oxygen are 1.0 and 0, respectively. The results are helpful for the recycle of magnesium sulfite in magnesia desulfurization.
Oxidation of magnesium sulfite is a crucial step in the wet magnesia desulfurization process. In this study, a green and robust solid catalyst, a SBA-15 (SBA, Santa Barbara Amorphous)-supported cobalt catalyst, was developed to promote the oxidation of sulfite. Both Co(III) and Co(II) served as the active sites for the oxidation of sulfite and were mainly located in the inner pore of the SBA-15.
Search
Popular Posts